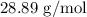Answer:
Molecular mass,
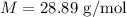
Step-by-step explanation:
Given that,
Mass of gas, m = 0.46 g
Volume of the container, V = 515 cm^3
Pressure, P = 153 kPa
Temperature, T = 322 K
We need to find the molecular mass of this gas. We know that,
PV =nRT
n = no of moles
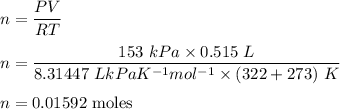
No of moles = mass/molecular mass
Let molecular mass is M
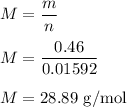
So, the molecular mass of this gas is