Answer and Explanation: Many nuclei are radioactive, which means they emit particles to become stable. In the process, they also become a different element. There are 3 types of decay:
- Alpha decay: it emits a particle of Helium, i.e., emits a particle with 2 as atomic number (Z) and 4 as atomic mass (A);
- Beta decay: emits an electron: a particle with 0 mass and -1 as atomic number;
- Gamma decay: emits a high energy form of electromagnetic radiation and it is extremely dangerous and penetrating;
Problem #1:
1.) Astatine: Z = 85 and A = 211
Alpha decay:
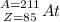 ⇒
⇒
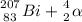
The isotope created is Bismuth
Characteristics: Z = 83; e⁻ = 83; n = A - Z = 207 - 83 = 124
The isotope is Bismuth with 83 protons, 83 electrons and 124 neutrons.
Problem #2
1.) Lead: Z = 82 and A = 82
Beta decay:
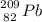 ⇒
⇒
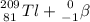
The isotope created is talium.
2.)
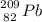 ⇒
⇒
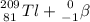
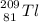 ⇒
⇒
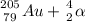
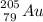 ⇒
⇒
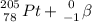
3.) The isotope created is
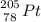 .
.
p⁺ = 78; e⁻ = 78; n = 127
The isotope created ahs 78 protons, 78 electrons and 127 neutrons.