Answer:
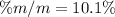
Step-by-step explanation:
Hello,
In this case given the molal solution of sucrose, we can assume there are 0.329 moles of sucrose in 1 kg of solvent, thus, computing both the mass of sucrose and solvent in grams, we obtain:
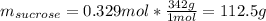
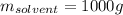
In such a way, we proceed to the calculation of the mass percent as follows:
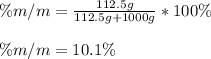
Regards.