Answer:
The answer is option B
Step-by-step explanation:
The denisty of a substance can be found by using the formula
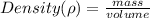
Making volume the subject we have
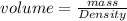
From the question
Density = 13.6 g/cm³
mass = 1.50 lbs
We must first convert the lbs to grams in order to solve
That's
if 2.2 lbs = 1 kg
Then 1.50 lbs =
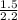
= 0.681 kg
If 1 kg = 1000 g
Then 0.68 kg = 0.681 × 1000 = 681g
So the mass = 681 g
Substitute the values into the above formula and solve for the volume
That's
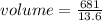
= 50.0735
We have the final answer as
50.1 mL
Hope this helps you