Answer:
There is 55.9 mL of acid in the solution.
Explanation:
We have:
V = 354 mL
%(a): concentration of acid = 15.8 %
Hence, we can find the number of milliliters of acid as follows:
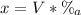
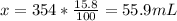
Therefore, there is 55.9 mL of acid in the solution.
I hope it helps you!