Given :
Mass of helium sample ,
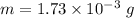 .
.
Density of helium ,
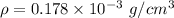 .
.
To Find :
The volume in millilitres .
Solution :
Density is given by ,
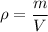
So , volume is ,
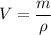
Putting given values in above equation we get :
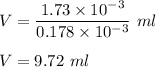
Therefore , volume of sample of helium is 9.72 ml .
Hence , this is the required solution .