Answer:
(a) 2.75 fm
(b) 2.89 fm
(c) 4.70 fm
(d) 7.12 fm
Step-by-step explanation:
For a given element, the radius r of its nuclei is given by;
r = r₀
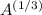
Where;
A = Atomic mass of the element
r₀ = 1.2 x 10⁻¹⁵m = 1.2fm
Now let's solve for the given elements
(a) ¹²₆C
Carbon element => This has an atomic mass number of 12
Therefore its radius is given by;
r = 1.2 x
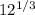
r = 1.2 x 2.29
r = 2.75 fm
(b) ¹⁴₇N
Nitrogen element => This has an atomic mass number of 14
Therefore its radius is given by;
r = 1.2 x
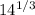
r = 1.2 x 2.41
r = 2.89 fm
(c) ⁶⁰₂₇Co
Cobalt element => This has an atomic mass number of 60
Therefore its radius is given by;
r = 1.2 x
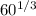
r = 1.2 x 3.92
r = 4.70 fm
(d) ²⁰⁸₈₂Pb
Lead element => This has an atomic mass number of 208
Therefore its radius is given by;
r = 1.2 x
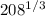
r = 1.2 x 5.93
r = 7.12 fm