Answer:
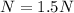
Step-by-step explanation:
Hello,
In this case, since normality is defined in terms of the equivalent grams and the volume of the solution:
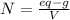
We need to compute the equivalent grams by considering that the molar mass of NaOH is 40 g/mol and one mole of NaOH contains one equivalent grams due to the presence of one hydroxyl ion only:
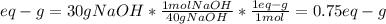
And the volume in liters (0.500 L). Therefore, we obtain:
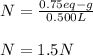
Regards.