Answer:
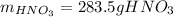
Step-by-step explanation:
Hello,
In this case, since the molarity is defined in terms of the moles of solute and the volume of solution in liters as follows:
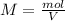
In such a way, solving for the moles of nitric acid, we obtain:
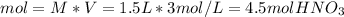
Finally, since the molar mass of nitric acid is 63 g/mol, the mass is computed as:
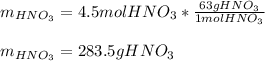
Regards.