Answer:
The change in temperature is
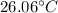 .
.
Step-by-step explanation:
It is given that,
The specific heat of Aluminium is cal/g°C
Mass of the sample, m = 4.55 g
Heat absorbed, Q = 2.55 cal
We need to find the change in temperature of the sample. The heat absorbed by an object is given by :
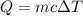
 is the change in temperature
is the change in temperature
So,
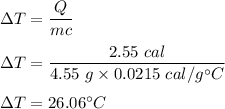
So, the change in temperature is
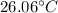 .
.