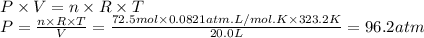Answer:
96.2 atm
Step-by-step explanation:
Step 1: Write the balanced equation
2 Na(s) + 2 H₂O(l) → 2 NaOH(aq) + H₂(g)
Step 2: Calculate the moles corresponding to 3.34 kg of sodium
The molar mass of Na is 22.99 g/mol.
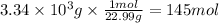
Step 3: Calculate the moles of hydrogen formed from 145 moles of sodium
The molar ratio of Na to H₂ is 2:1. The moles of H₂ formed are 1/2 × 145 mol = 72.5 mol
Step 4: Convert the temperature to the Kelvin scale
K = °C + 273.15 = 50.0°C + 273.15 = 323.2 K
Step 5: Calculate the pressure exerted by the hydrogen gas
We will use the ideal gas equation.