Answer:
0 kJ/mol.
Step-by-step explanation:
Hello,
In this case, since the chemical potential can be represented in terms of the Gibbs free energy of formation:
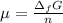
Thus, since the Gibbs free energy of formation of an element is zero, the chemical potential is also zero, or just 0 kJ/mol.
Best regards.