Answer:
a) 50%
b) 100 J
c) 50 J
Step-by-step explanation:
The cold temperature of the reservoir =
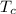
according to the problem, it is stated that the hot reservoir of an engine is twice that of the cold reservoir, therefore,
the hot temperature of the reservoir
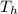 =
=
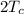
The work done by the engine = 50 J
a) The max efficiency obtainable from a heat engine η =
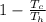
since
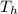 =
=
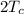 , the equation becomes
, the equation becomes
η =
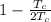 =
=
η =
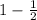 = 0.5 = 50%
= 0.5 = 50%
b) The heat absorbed per cycle will be gotten from
η =
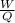
η is the efficiency of the system = 0.5
where W is the work done = 50 J
Q is the heat absorbed = ?
substituting, we have
0.5 =
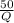
Q = 50/0.5 = 100 J
c) The heat rejected per cycle = 50% of the absorbed heat
==> 0.5 x 100 J = 50 J