Answer:
0.228 nm
Step-by-step explanation:
Atomic mass number of copper = 64
but an atomic mass unit = 1.66 x 10^-27 kg
therefore, the mass of the copper atom m = 64 x 1.66 x 10^-27 kg = 1.06 x 10^-25 kg
The number of atoms in this mass n = ρ/m
where ρ is the density of copper = 8.96 x 10^3 kg/m^3
==> n = (8.96 x 10^3)/(1.06 x 10^-25) = 8.45 x 10^28 atoms/m^3
We know that the volume occupied by this amount of atoms n =
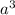
where a is the lattice constant
equating, we have
8.45 x 10^28 =
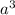
a = 4.389 x 10^9
we also know that
d = 1/a
where d is the smallest distance between the two copper atom.
d = 1/(4.389 x 10^9) = 2.28 x 10^-10 m
==> 0.228 nm