Answer:
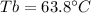
Step-by-step explanation:
Hello,
In this case, for the boiling point elevation, we have:

Whereas the van't Hoff factor for the given substance is 1 since is not ionizing. Moreover, the molality is computed by:
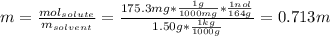
In such a way, since the boiling point of pure chloroform is 61.2 °C, the boiling point of the solution is:
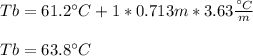
Regards.