Answer:
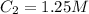
Step-by-step explanation:
Hello,
In this case, since the concentration in #1 is 3M, during a dilution process, the moles of the solute (NaCl) remains the same, just the concentration and volume change as shown below:
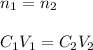
In such a way, as the final volume is 1200 microliters, the resulting concentration turns out:
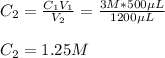
Best regards.