Answer:
![\text{Rate} = k\, [\mathrm{H_2\, (g)}]\cdot [\mathrm{NO\, (g)}]^2](https://img.qammunity.org/2021/formulas/chemistry/college/sqfjw80rqqswiypi0tu2u1z2dwb3jeqnrq.png) .
.
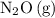 is the intermediate.
is the intermediate.
Step-by-step explanation:
Rate
Balanced overall reaction:
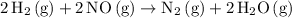 .
.
Proposed mechanism:
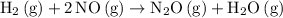 (slow.)
(slow.)
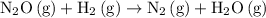 (fast.)
(fast.)
Start with the slowest, rate-determining step of the proposed mechanism. Here, the rate-determining step is
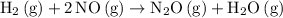 .
.
There are two species on the reactant side of this intermediate reaction:
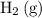 and
and
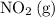 . The concentration of both of them should be in the rate expression of this step.
. The concentration of both of them should be in the rate expression of this step.
On the other hand, the coefficient of
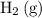 is one while the coefficient of
is one while the coefficient of
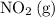 is two. Therefore, in the rate expression of this step, the concentration of
is two. Therefore, in the rate expression of this step, the concentration of
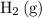 should have a power of one, while the concentration of
should have a power of one, while the concentration of
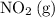 should have a power of two.
should have a power of two.
include the rate constant
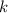 to obtain the rate expression of the rate-determining slow step:
to obtain the rate expression of the rate-determining slow step:
![\text{Rate} = k\, [\mathrm{H_2\,(g)}]\cdot [\mathrm{NO\, (g)}]^2](https://img.qammunity.org/2021/formulas/chemistry/college/1dub75n9juhkbi3wucmbchtmz3sz0vgfzx.png) .
.
Make sure that all species in this rate expression are on the reactant side of the overall balanced reaction. Otherwise, further steps would be required to obtain the rate law of the overall reaction.
Therefore, by this proposed mechanism, the rate law of the overall reaction would be
![\text{Rate} = k\, [\mathrm{H_2\,(g)}]\cdot [\mathrm{NO\, (g)}]^2](https://img.qammunity.org/2021/formulas/chemistry/college/1dub75n9juhkbi3wucmbchtmz3sz0vgfzx.png) .
.
Intermediate
In a proposed reaction mechanism, a species is an intermediate if it appeared in one of the proposed steps, but not in the balanced overall equation.
The two steps of this proposed mechanism mentioned five species:
With the exception of
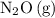 , all the other species appeared in the overall balanced equation. Therefore,
, all the other species appeared in the overall balanced equation. Therefore,
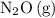 is the intermediate.
is the intermediate.