Answer:
The pH of the solution will be 3
Step-by-step explanation:
The strength of acids is determined by their ability to dissociate into ions in aqueous solution. A strong acid is any compound capable of completely and irreversibly releasing protons or hydrogen ions, H⁺. That is, an acid is said to be strong if it is fully dissociated into hydrogen ions and anions in solution.
Being pH=- log [H⁺] or pH= - log [H₃O⁺] and being a strong acid, all the HClO₃ dissociates:
HClO₄ + H₂O → H₃O⁺ + ClO₄-
So: [HCLO₄]= [H₃O⁺]
The molar concentration is:
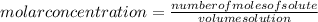
The molar mass of HClO₄ being 100 g / mole, then if 100 grams of the compound are present in 1 mole, 0.025 grams in how many moles are present?
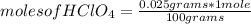
moles of HClO₄= 0.00025
Then:
![[HClO_(4)]=(0.00025 moles)/(0.25 L)](https://img.qammunity.org/2021/formulas/chemistry/college/4djf00u6zt1lwvb7owlsqqw06ki7qqoiqr.png)
![[HClO_(4)]=0.001 ( moles)/( L)](https://img.qammunity.org/2021/formulas/chemistry/college/barztyl9bnjggxzwca5736x6a1xjhkop3c.png)
Being [HCLO₄]= [H₃O⁺]:
pH= - log 0.001
pH= 3
The pH of the solution will be 3