Answer:
C. 0.191 M
Step-by-step explanation:
Our goal for this question, is to calculate the concentration of the HCl solution. For this, in the experiment, a solution of NaOH was used to find the moles of HCl. Therefore, our first step is to know the reaction between HCl and NaOH:
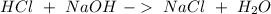
The "titrant" in this case is the NaOH solution. If we know the concentration of NaOH (0.100M) and the volume of NaOH (38.2 mL=0.0382 L), we can calculate the moles using the molarity equation:
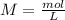
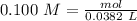
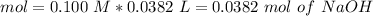
Now, in the reaction, we have a 1:1 molar ratio between HCl and NaOH (1 mol of HCl is consumed for each mole of NaOH added). Therefore we will have the same amount of moles of HCl in the solution:
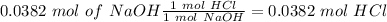
If we want to calculate the molarity of the HCl solution we have to divide by the litters of HCl used in the experiment (20 mL= 0.02 L):
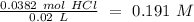
The concentration of the HCl solution is 0.191 M
I hope it helps!