Answer:
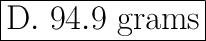
Step-by-step explanation:
Phosphate chemical formula is given as,
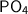
There is one phosphorus atom and four oxygen atoms.
Atomic mass of Phosphorus
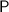 = 30.974 grams
= 30.974 grams
Atomic mass of Oxygen
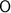 = 15.999 grams
= 15.999 grams
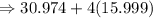
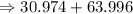
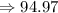
The relative formula mass of phosphate
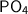 is 94.9 grams.
is 94.9 grams.