Answer:
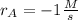
Step-by-step explanation:
Hello,
In this case, given the rate of production of C, we can compute the rate of consumption of A by using the rate relationships which include the stoichiometric coefficients at the denominators (-1 for A and 2 for C) as follows:
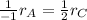
In such a way, solving the rate of consumption of A, we obtain:
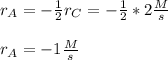
Clearly, such rate is negative which account for consumption process.
Regards.