Answer:
5.74M or 5.74 mol/L (to 3 sign. fig.)
Step-by-step explanation:
The molar mass of NaNO3 is 85g/mol, which means that:
1 mole of NaNO3 - 85g
? moles - 122.0g
= 122/85 = 1.44 moles
Concentration in mol/L = no. of moles (moles) ÷ volume (L)
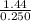 = 5.74M or 5.74 mol/L (to 3 sign. fig.)
= 5.74M or 5.74 mol/L (to 3 sign. fig.)
I hope the steps are clear and easy to follow.