Answer:
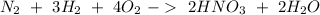
Step-by-step explanation:
We can start with the reaction of hydrogen and nitrogen to produce ammonia, so:
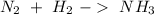
When we balance the reaction we will obtain:
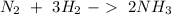
Now, the production of nitric acid with oxygen would be:
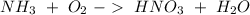
If we balance the reaction we will obtain:
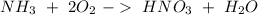
Now, if we put the reactions together we will obtain:
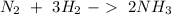
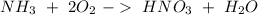
We can multiply the second reaction by "2":
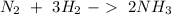
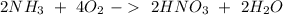
We have "
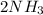 " on both sides. In the first reaction is in the right in the second reaction is on the left. Therefore we can cancel out this compound and we will obtain:
" on both sides. In the first reaction is in the right in the second reaction is on the left. Therefore we can cancel out this compound and we will obtain:
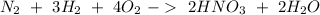
On this reaction, we will have 2 nitrogen atoms on both sides, 6 hydrogen atoms on both sides, and 8 oxygen atoms on both sides. So, this would be the net reaction for the production of nitric acid.