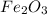Answer:
1)
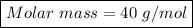
2)
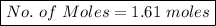
3)
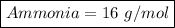
4)
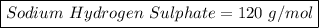
5)
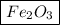
Step-by-step explanation:
Question # 1:
Using Formula, No. of moles = Mass in grams / molar mass
Where moles = 0.375 mol. , mass = 15 g
=> Molar Mass = Mass in grams / No. of moles
=> Molar Mass = 15 / 0.375
=> Molar mass = 40 g/mol
Question # 2:
No. of moles = Mass in grams / Molar mass
Where Mass = 90 g ,
=> Molar Mass =
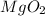
=> MM = 24 + (16*2)
=> MM = 24 + 32
=> MM = 56 g/mol
No. of Moles = 90 / 56
No. of Moles = 1.61 moles
Question # 3:
Ammonia =>
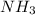
N has atomic mass 14 ang H has atomic mass 1
Ammonia = (14)+(1*2)
Ammonia = 14+2
Ammonia = 16 g/mol
Question # 4:
Sodium Hydrogen Sulphate =>
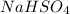
Where Na has atomic mass 23, H has atomic mass 1 , sulpher 32 and oxygen 16
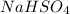 = 23+1+32+(16*4)
= 23+1+32+(16*4)
=> 56+64
=> 120 g/mol
Question # 5:
The metal which has relative atomic mass of 56 is Iron (Fe)
Given that the oxide contains 70.0 % of Metal
Mass of
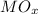 (Metal oxide) = 56 / 70 * 100
(Metal oxide) = 56 / 70 * 100
Mass of
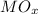 (Metal oxide) = 0.8 * 100
(Metal oxide) = 0.8 * 100
Mass of
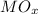 (Metal oxide) = 80
(Metal oxide) = 80
Mass of x O's = 80 - 56
Mass of x O's = 24
Now, Mass of O = 24 / 16
Mass of O = 1.5
So, Fe Metal and its oxide are in the ratio of
1 : 1.5
×2 ×2
2 : 3
So, the empirical formula of metal with its oxide is