Answer:
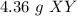
Step-by-step explanation:
In this case, we can start with the reaction:
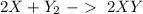
If we check the reaction, we will have 2 X and Y atoms on both sides. So, the reaction is balanced. Now, the problem give to us two amounts of reagents. Therefore, we have to find the limiting reagent. The first step then is to find the moles of each compound using the molar mass:
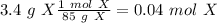
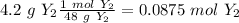
Now, we can divide by the coefficient of each compound (given by the balanced reaction):
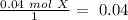
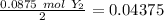
The smallest value is for "X", therefore this is our limiting reagent. Now, if we use the molar ratio between "X" and "XY" we can calculate the moles of XY, so:
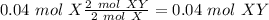
Finally, with the molar mass of "XY" we can calculate the grams. Now, we know that 1 mol X = 85 g X and 1 mol
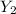 = 48 g
= 48 g
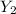 (therefore 1 mol Y = 24 g Y). With this in mind the molar mass of XY would be 85+24 = 109 g/mol. With this in mind:
(therefore 1 mol Y = 24 g Y). With this in mind the molar mass of XY would be 85+24 = 109 g/mol. With this in mind:
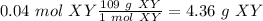
I hope it helps!