Answer:
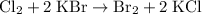 .
.
One chlorine molecule reacts with two formula units of (aqueous) potassium bromide to produce one bromine molecule and two formula units of (aqueous) potassium chloride.
Step-by-step explanation:
Formula for each of the species
Start by finding the formula for each of the compound.
- Both chlorine
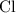 and bromine
and bromine
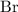 are group 17 elements (halogens.) Each
are group 17 elements (halogens.) Each - On the other hand, potassium
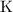 is a group 1 element (alkaline metal.) Each
is a group 1 element (alkaline metal.) Each
Therefore, the ratio between
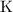 atoms and
atoms and
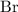 atoms in potassium bromide is supposed to be one-to-one. That corresponds to the empirical formula
atoms in potassium bromide is supposed to be one-to-one. That corresponds to the empirical formula
 . Similarly, the ratio between
. Similarly, the ratio between
The formula for chlorine gas is
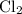 , while the formula for bromine gas is
, while the formula for bromine gas is
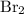 .
.
Balanced equation for the reaction
Write down the equation using these chemical formulas.
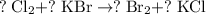 .
.
Start by assuming that the coefficient of compound with the largest number of elements is one. In this particular equation, both
 and
and
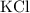 features two elements each.
features two elements each.
Assume that the coefficient of
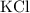 is one. Hence:
is one. Hence:
 .
.
Note that
 is the only source of
is the only source of
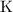 and
and
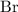 atoms among the reactants of this reaction.
atoms among the reactants of this reaction.
There would thus be one
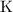 atom and one
atom and one
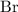 atom on the reactant side of the equation.
atom on the reactant side of the equation.
Because atoms are conserved in a chemical equation, there should be the same number of
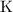 and
and
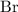 atoms on the product side of the equation.
atoms on the product side of the equation.
In this reaction,
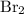 is the only product with
is the only product with
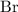 atoms.
atoms.
One
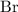 atom would correspond to
atom would correspond to
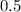 units of
units of
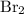 .
.
Similarly, in this reaction,
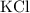 is the only product with
is the only product with
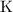 atoms.
atoms.
One
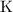 atom would correspond to one formula unit of
atom would correspond to one formula unit of
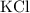 .
.
Hence:
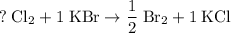 .
.
Similarly, there should be exactly one
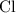 atom on either side of this equation. The coefficient of
atom on either side of this equation. The coefficient of
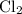 should thus be
should thus be
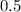 . Hence:
. Hence:
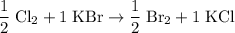 .
.
That does not meet the requirements, because two of these coefficients are not integers. Multiply all these coefficients by two (the least common multiple- LCM- of these two denominators) to obtain:
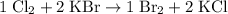 .
.