Answer:
Hello the needed relation is missing below is the required relation
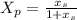 composition : propane = 0.70, butane = 0.3
composition : propane = 0.70, butane = 0.3
Answer : ≈ 5.75 hrs
Step-by-step explanation:
Applying the data given in regards to the material balance
Butane balance input into the still = 5 mole feed/hr | 0.30 mol butane/molfeed
since the total volume of the liquid in the still is constant
The output from the still is = 5mol condensed/hr | x
 mol butane/mol condensed
mol butane/mol condensed
unsteady state equation =
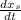 = 0.15 -
= 0.15 -
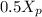
note : to reduce the equation a single dependent variable we have to substitute for
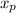
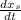
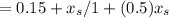
In order to find the time it will take for X to change from 0.3 to 0.35
integrate the above equation using the limits : t = 0, x
 = 0.3 and t = Ф,
= 0.3 and t = Ф,
x
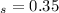
=
![[ - (x_(s) /0.35 - (1/(0.35)^2)* In(0.15 - 0.35x_(s) ) ]_(0.3) ^(0.35)](https://img.qammunity.org/2021/formulas/engineering/college/4vkcfqwmi2a87ogbpr85mlku74mgmufy1f.png)
hence t = Ф ≈ 5.75 hrs