Answer:
≈ 76.16%
Step-by-step explanation:
What is Percent Yield? In chemistry, percent yield is the percent ratio of the weight of the product obtained to the theoretical yield. We calculate the percent yield by dividing the experimental yield by the theoretical yield and multiplying the result by 100 to express the final answer in %.
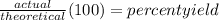 ⇒
⇒
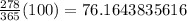
≈ 76.16%
hope this helped :)