Answer:
D) two unhybridized p orbitals
Step-by-step explanation:
In covalent bond, to form a bond, each of the two participating atoms would put down an unpaired electron to be used in forming a shared pair of electrons between them.
There are two types of covalent bonds:
A sigma bond
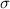
A pi bond
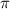
A sigma bond is formed when a hybrid orbital (sp,sp² and sp³) overlaps with another hybrid orbital or with s- or p- orbital.
A pi bond is formed when a p-orbital overlaps with another parallel p-orbital laterally. This implies that ,a π bond could be formed from the overlap of two unhybridized p orbitals.