Answer:
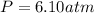
Step-by-step explanation:
Hello,
In this case, we can study the ideal gas equation that relates temperature, volume, pressure and moles as shown below:

Thus, since we are asked to compute the pressure y simply solve for it as follows:
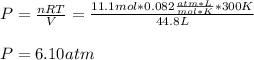
Best regards.