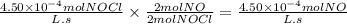Answer:
4.50 × 10⁻⁴ mol L⁻¹ s⁻¹
Step-by-step explanation:
Step 1: Write the balanced equation
2 NO(g) + Cl₂(g) → 2 NOCl(g)
Step 2: Establish the appropriate molar ratio
The molar ratio of NO(g) to NOCl(g) is 2:2, that is, when 2 moles of NO(g) are consumed, 2 moles of NOCl(g) are formed.
Step 3: Calculate the rate of consumption of NO(g)
The rate of formation of NOCl(g) is 4.50 × 10⁻⁴ mol L⁻¹ s⁻¹. The rate of consumption of NO(g) is: