Answer:

The reactant that is reduced is
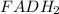
Step-by-step explanation:
The complete equation is as below:

Recall that oxidation involves the gain of electrons while reduction involves the loss of electrons.
In the above reaction,
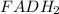 loses electrons to coenzyme Q and becomes reduced to FAD, hence the oxidizing agent. Coenzyme Q gains electrons and becomes oxidized to
loses electrons to coenzyme Q and becomes reduced to FAD, hence the oxidizing agent. Coenzyme Q gains electrons and becomes oxidized to
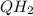 , hence the reducing agent.
, hence the reducing agent.
In order words,
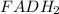 is reduced while coenzyme Q is oxidized.
is reduced while coenzyme Q is oxidized.