Answer:
It will take 6486.92 minutes for [H2O2] to fall from 0.95 M to 0.33 M
Step-by-step explanation:
The order of reaction is defined as the sum of the powers of the concentration terms in the equation. Order of a reaction is given by the number of atoms or molecule whose concentration change during the reaction and determine the rate of reaction.
In first order reaction;
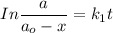
where;
a = concentration at time t
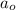 = initial concentration
= initial concentration
and k = constant.
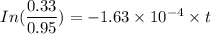
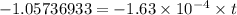
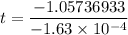
t = 6486.92 minutes