Answer:
the melting point T = 125.36°C
Step-by-step explanation:
Given that:
The resistance of a platinum thermometer at 0°C is
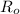 = 160.0 ohms
= 160.0 ohms
The resistance of a platinum thermometer when immersed in a crucible containing a melting substance
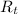 = 243.8 ohms
= 243.8 ohms
The temperature coefficient at room temperature 20°C = ∝ = 0.00392
The objective is to determine the melting point of this substance
To do that ; at 20°C, the resistance of the platinum thermometer can be calculated as follows:
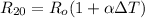
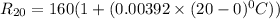
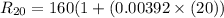
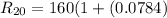
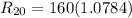
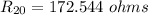
The resistance of the platinum thermometer at t°C ,
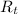 =
=
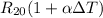
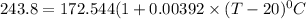
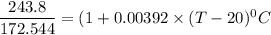
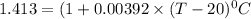
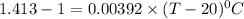
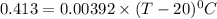
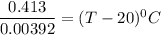
105.36°C = (T - 20) °C
T = 105.36°C + 20 °C
T = 125.36°C