Answer:
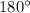 by the VSEPR theory.
by the VSEPR theory.
Step-by-step explanation:
This question is asking for the bond angle of the
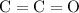 bond in
bond in
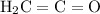 . The VSEPR (valence shell electron pair repulsion) theory could help. Start by considering: how many electron domains are there on the carbon atom between these two bond?
. The VSEPR (valence shell electron pair repulsion) theory could help. Start by considering: how many electron domains are there on the carbon atom between these two bond?
Note that "electron domains" refer to covalent bonds and lone pairs collectively.
- Each nonbonding pair (lone pair) of valence electrons counts as one electron domain.
- Each covalent bond (single bond, double bond, or triple bond) counts as exactly one electron domain.
For example, in
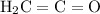 , the carbon atom at the center of that
, the carbon atom at the center of that
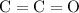 bond has two electron domains:
bond has two electron domains:
- This carbon atom has two double bonds: one
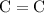 bond and one
bond and one
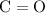 bond. Even though these are both double bonds, in VSEPR theory, each of them count only as one electron domain.
bond. Even though these are both double bonds, in VSEPR theory, each of them count only as one electron domain. - Keep in mind that there are only four valence electrons in each carbon atom. It can be shown that all four valence electrons of this carbon atom are involved in bonding (two in each of the two double bonds.) Hence, there would be no nonbonding pair around this atom.
In VSEPR theory, electron domains around an atom repel each other. As a result, they would spread out (in three dimensions) as far away from each other as possible. When there are only two electron domains around an atom, the two electron domains would form a straight line- with one domain on each side of the central atom. (To visualize, consider the three atoms in this
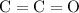 bond as three spheres on a stick. The central
bond as three spheres on a stick. The central
 atom would be between the other
atom would be between the other
 atom and the
atom and the
 atom.)
atom.)
This linear geometry corresponds to a bond angle of
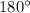 .
.