Answer:
Joseph Henry.
Step-by-step explanation:
Hello,
In this case, we define the accuracy based on the degree of deviation a measure has regarding the the true or expected value. In this case, the lower the difference, the more accurate the procedure. In such a way, for the two students, we compute the difference between the expected value and the obtained value:
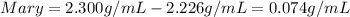
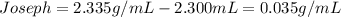
In such a way, since the difference is lower in Joseph's measure, we can say he is the most accurate.
Best regards.