Answer:
The volume percent is 8.71 % v/v
Step-by-step explanation:
The volume percent (v/v) of the solution can be found using the following equation:
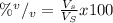 (1)
(1)
Where:
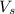 : is the solute volume = 28.3 mL
: is the solute volume = 28.3 mL
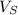 : is the solution volume = 325 mL
: is the solution volume = 325 mL
By introducing the above values into equation (1) we have the volume percent:
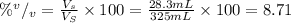
Therefore, the volume percent is 8.71 % v/v.
I hope it helps you!