Answer:
See explanation
Step-by-step explanation:
The reaction with
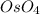 and
and
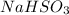 will add two "OH" groups to the molecule. In the reaction (figure 1) we can see the different structures for the alkenes. In the Z alkene (cis) we have the 2 methyl groups on the same side. In the E alkene (trans) the 2 methyl groups are placed on opposite sides. In the products, all the "OH" groups are placed at the top. This indicates that the addition of the hydroxyl groups is "syn". In the syn reactions, all the groups are bonded on the same side therefore we will have a stereospecific reaction.
will add two "OH" groups to the molecule. In the reaction (figure 1) we can see the different structures for the alkenes. In the Z alkene (cis) we have the 2 methyl groups on the same side. In the E alkene (trans) the 2 methyl groups are placed on opposite sides. In the products, all the "OH" groups are placed at the top. This indicates that the addition of the hydroxyl groups is "syn". In the syn reactions, all the groups are bonded on the same side therefore we will have a stereospecific reaction.
I hope it helps!