Answer:
the temperature of the aluminum at this time is 456.25° C
Step-by-step explanation:
Given that:
width w of the aluminium slab = 0.05 m
the initial temperature
 = 25° C
= 25° C
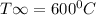
h = 100 W/m²
The properties of Aluminium at temperature of 600° C by considering the conditions for which the storage unit is charged; we have ;
density ρ = 2702 kg/m³
thermal conductivity k = 231 W/m.K
Specific heat c = 1033 J/Kg.K
Let's first find the Biot Number Bi which can be expressed by the equation:
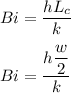
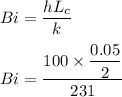
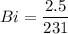
Bi = 0.0108
The time constant value
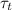 is :
is :
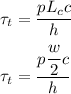
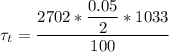
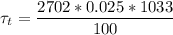
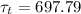
Considering Lumped capacitance analysis since value for Bi is less than 1
Then;
![Q= (pVc)\theta_1 [1-e^{\frac {-t}{ \tau_1}}]](https://img.qammunity.org/2021/formulas/engineering/college/5gi7b5ml8pq80xb2o21a0dg3ql9zryrrla.png)
where;
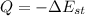 which correlates with the change in the internal energy of the solid.
which correlates with the change in the internal energy of the solid.
So;
![Q= (pVc)\theta_1 [1-e^{\frac {-t}{ \tau_1}}]= -\Delta E _(st)](https://img.qammunity.org/2021/formulas/engineering/college/x8r8fldut2tye0gu7kt95xq32enlofyw6t.png)
The maximum value for the change in the internal energy of the solid is :
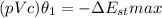
By equating the two previous equation together ; we have:
![\frac{-\Delta E _(st)}{\Delta E _(st){max}}= \frac{ (pVc)\theta_1 [1-e^{\frac {-t}{ \tau_1}}]} { (pVc)\theta_1}](https://img.qammunity.org/2021/formulas/engineering/college/4mb77wcitedjmqu9k1clnwwganaibfx4lr.png)
Similarly; we need to understand that the ratio of the energy storage to the maximum possible energy storage = 0.75
Thus;
![0.75= [1-e^{\frac {-t}{ \tau_1}}]}](https://img.qammunity.org/2021/formulas/engineering/college/8lvvonv0gytp7xwmhekgms2kgqlpf8xxe8.png)
So;
![0.75= [1-e^{\frac {-t}{ 697.79}}]}](https://img.qammunity.org/2021/formulas/engineering/college/azbvs1ki8bjwauyu2r1wycqixijd6bvlm6.png)
![1-0.75= [e^{\frac {-t}{ 697.79}}]}](https://img.qammunity.org/2021/formulas/engineering/college/dioyo6yee1rjho4vnottjq4izuqzm592gh.png)
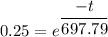
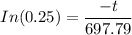
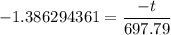
t = 1.386294361 × 697.79
t = 967.34 s
Finally; the temperature of Aluminium is determined as follows;
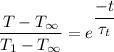
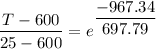
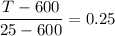
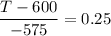
T - 600 = -575 × 0.25
T - 600 = -143.75
T = -143.75 + 600
T = 456.25° C
Hence; the temperature of the aluminum at this time is 456.25° C