Answer:
The work done by the carnot engine per cycle is 1318.31 J
Step-by-step explanation:
Given;
high temperature reservoir, Th = 435 k
temperature of river water, Tl = 280 k
heat energy absorbed per cycle, Q = 3700 J
Determine the work done per cycle is calculated as;
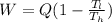
Where;
W is the work done
Q is the absolute heat absorbed per cycle
Tl is the temperature of the cold liquid
Th is the temperature of the hot reservoir
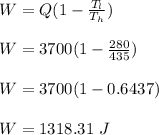
Therefore, the carnot engine operating at the given conditions, performs 1318.31 J work per cycle.