Answer:
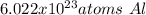
Step-by-step explanation:
Hello,
In this case, given the described concept regarding the Avogadro's number, we can easily notice that 27.0 g of aluminium foil has 6.022x10²³ atoms as shown below based on the mass-mole-particles relationship:
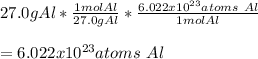
Notice this is backed up by the fact that aluminium molar mass if 27.0 g/mol.
Best regards.