Answer:
See explanation
Step-by-step explanation:
In this case, we can start with the calculation of the total energy released by the 20 matches. One match release 838.2 J of energy ( 1 match = 838.2 J). So:
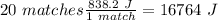
With this in mind, we have to find al the conversion ratios to "Jolues", so:
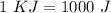
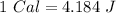
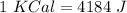
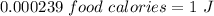
Now, we can do the conversions:
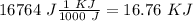
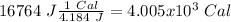
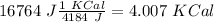
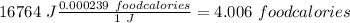
I hope it helps!