Answer:
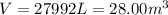
Step-by-step explanation:
Hello,
In this case, the combustion of methane is shown below:
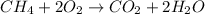
And has a heat of combustion of −890.8 kJ/mol, for which the burnt moles are:
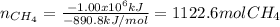
Whereas is consider the total released heat to the surroundings (negative as it is exiting heat) and the aforementioned heat of combustion. Then, by using the ideal gas equation, we are able to compute the volume at 25 °C (298K) and 745 torr (0.98 atm) that must be measured:
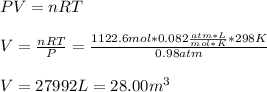
Best regards.