Answer:
The HCl is very strong since its pH is equal to 0.17.
Step-by-step explanation:
The reaction between CaCO₃ and HCl is:
CaCO₃(s) + 2HCl(aq) ⇄ CaCl₂(aq) + CO₂(g) + H₂O(l) (1)
The number of moles of CaCO₃ is:
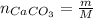
Where:
m: is the mass = 0.750 g
M: is the molar mass = 100.0869 g/mol
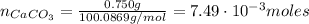
From the reaction (1) we have that 1 mol of CaCO₃ reacts with 2 moles of HCl, so the number of moles of HCl is:
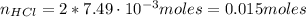
Now, with the number of moles of HCl we can find its concentration:
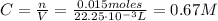
Finally, the pH of the acid is:
![pH = -log([H^(+)]) = -log(0.67) = 0.17](https://img.qammunity.org/2021/formulas/chemistry/college/ch4bpe413ta6rychtp7mqa7zgob28k0amt.png)
The pH obtained is very low, so the HCl is very strong.
Therefore, the HCl is very strong since its pH is equal to 0.17.
I hope it helps you!