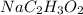Answer: (i)
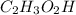 and (ii)
and (ii)
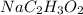
Step-by-step explanation:
Buffer is a solution which resists changes in pH when small quantities of acid or alkali are added to it.
Acidic buffer is formed by combination of weak acid and salt of weak acid with strong base. Example:
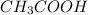 and
and
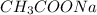
Basic buffer is formed by combination of weak base and salt of weak base with strong acid. Example:
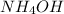 and
and
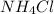
Thus pair of substance can be dissolved together (in the right ratio) to prepare a buffer solution is
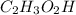 and
and