Answer:
See explanation
Step-by-step explanation:
Our answer options for this question are:
a. 2-chlorobutanoic acid:_______ 2-chlorobutanoic acid:_______ 3-chlorobutanoic acid:______.
b. 2,4-dinitrobenzoic acid:______ p-nitrobenzoic acid:______ p-bromobenzoic acid:_______.
c. p-cyanobenzoic acid:________ benzoic acid:_______ p-aminobenzoic acid:______
We have to check each set of molecules
a. 2-chlorobutanoic acid, 3-chlorobutanoic acid
In this case, the difference between these molecules is the position of "Cl". If the chlorine atom is closer to the acid group, we will have a higher inductive effect. So, the bond O-H would be weaker and we will have more acidity. So, the molecule with more acidity is 2-chlorobutanoic acid and the less acidic would be 3-chlorobutanoic acid.
b. 2,4-dinitrobenzoic acid, p-nitrobenzoic acid, p-bromobenzoic acid
In this case, we have several structural differences. In all the structure, we have deactivating groups (
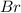 and
and
 ). If we have a deactivating group the acidity will increase. In the case of "Br", we have a weak deactivating, so, this will be the less acidic one (p-bromobenzoic acid)
). If we have a deactivating group the acidity will increase. In the case of "Br", we have a weak deactivating, so, this will be the less acidic one (p-bromobenzoic acid)
in 2,4-dinitrobenzoic acid we have two deactivating groups, therefore, this would be the most acid compound.
c. p-cyanobenzoic acid, benzoic acid, p-aminobenzoic acid
On these molecules, we have several structural differences. In p-cyanobenzoic acid we have a deactivating group, therefore in this molecule we will have more acidity. In the p-aminobenzoic acid, we have an activating group, so, this would be the less acidic compound.
See figure 1
I hope it helps!