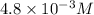Answer: The solubility of the salt is
Step-by-step explanation:
Solubility product is defined as the equilibrium constant in which a solid ionic compound is dissolved to produce its ions in solution. It is represented as

The equation for the reaction will be as follows:
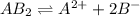
By Stoichiometry,
1 mole of
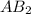 gives 1 mole of
gives 1 mole of
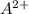 and 2 moles of
and 2 moles of
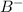
Thus if solubility of
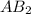 is s moles/liter, solubility of
is s moles/liter, solubility of
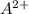 is s moles\liter and solubility of
is s moles\liter and solubility of
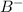 is 2s moles/liter
is 2s moles/liter
Therefore,
![K_sp=[A^(2+)][B^(-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/5x4c3b85fkg74zomq991kxk8ar23rqqq4n.png)
![4.3* 10^(-7)=[s][2s]^2](https://img.qammunity.org/2021/formulas/chemistry/college/cum1ukfr2rkprc7krnbucsx32j9zcuu256.png)
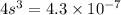

The solubility of the salt is