Answer:
so mass in gram=560grams
Step-by-step explanation:
number of moles=10moles
molar mass=56grams/moles
mass in gram of Fe=?
as we know that
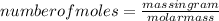
evaluating the formula
number of moles×molar mass=mass in gram
mass in gram=10moles×56grams/moles
mass in gram=560grams
i hope this will help you :)