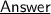
As we know,
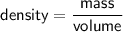
So, we can infer that :
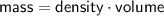
Now, let's calculate the mass of gases in each case :
Case A : Hydrogen ~
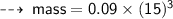
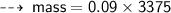
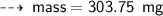
or
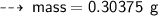
Case B : Helium ~
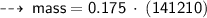
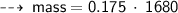
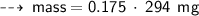
or
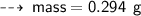
Case C : Nitrogen ~
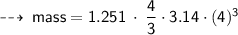
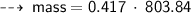
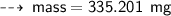
or
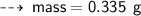
So, the arrangement of masses from least to greatest is :
- (1.) Hydrogen < (2.) Helium < (3.) Nitrogen