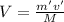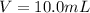Answer:
Take 10.0mL of stock solution using 10.0mL pipette in a 100mL volumetric flask and make up the solution with deionized water up to the mark of the flask and we get 100mL of 1.9×10⁻⁵M solution.
Step-by-step explanation:
Molarity of stock solution,
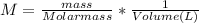
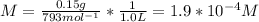
According to law of dilution MV = m'v'
Where, M = Molarity of stock solution = 1.9×10⁻⁴M
V = Volume of stock solution = ?
m' = Molarity of dilute solution = 1.9×10⁻⁵M
v' = Volume of dilute solution = 100 mL
Substituting the above parameters in the equation of dilution